
Features
Research
Recirc in Action: Research to evaluate peracetic acid for use in U.S. recirculating aquaculture
October 4, 2023 By Christopher Good, Freshwater Institute
 Figure 1. Twelve replicated experimental-scale fluidized sand biofilters used to examine the impacts of low-dose peracetic acid on nitrification performance.
Photos: Freshwater Institute
Figure 1. Twelve replicated experimental-scale fluidized sand biofilters used to examine the impacts of low-dose peracetic acid on nitrification performance.
Photos: Freshwater Institute As with other water quality parameters, bacteria and biofilms can accumulate in recirculating aquaculture systems (RAS) over time, and in some situations, disinfection efforts need to be applied to reduce waterborne microbial populations.
Effective, full-flow disinfection can be achieved through the combined use of ozonation and UV irradiation; however, equipment, operating, and maintenance costs must be considered with this approach. Chemical disinfectants applied to RAS water can be an alternative method, although the balance between antimicrobial efficacy and biofilter performance can be problematic, i.e., the necessary concentrations of specific chemicals for killing target organisms might also impact bacterial nitrification processes in the biofilter, leading to levels of ammonia and/or nitrite that are unsafe for fish. As such, low-dose approaches to chemical water disinfection have been an area of investigation in Europe and North America, to determine biofilter-friendly yet efficacious application procedures.
Peracetic acid (PAA) is one such water disinfectant that has received considerable attention, especially in Denmark, where RAS farmers have been seeking alternatives to formalin to control waterborne fish pathogen loads. Compared to formalin, which poses a carcinogenic risk to RAS operators, PAA is considered relatively safe and environmentally friendly, as it degrades to acetic acid, oxygen, and water in the aquatic environment, and does not impart toxic residues.
Danish researchers have demonstrated that low-dose applications of PAA can be efficacious for reducing RAS bacterial loads while not significantly impacting biofiltration.
Currently, PAA is approved for use in the European Union as a water disinfectant in aquaculture settings; however, until 2023 PAA was only approved in the U.S. for use as a surface disinfectant on fish farms and was not approved for administration while food fish were present. This year, a commercial PAA product (VIGOROX® Trident; Evonik, Piscataway, NJ) has finally been registered by the U.S. Environmental Protection Agency as a water disinfectant for use in RAS and ponds.
While this approval is good news for U.S. RAS operators, continuing research is necessary to refine and optimize PAA application protocols across a range of RAS environments, as it is well-known that PAA efficacy can be significantly influenced by differing water quality profiles.
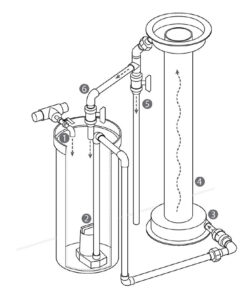
Figure 2. Schematic of an individual experimental-scale fluidized sand biofilter.
1. System influent.
2. Submersible pump.
3. Biofilter influent.
4. Fluidized sand bed. Post-biofilter
water flow can be sent to
5. Discharge, or
6. Recirculation within the biofilter system.
From 2015-2023, The Conservation Fund’s Freshwater Institute (Shepherdstown, WV) has been part of the Norwegian Research Council-funded CtrlAQUA program, hosted by Nofima and composed of a range of Norwegian and international research institutions and aquaculture industry partners.
The major objective of the CtrlAQUA program was to support the functional and economic viability of closed containment aquaculture through technological and biological research and innovation. The scope of CtrlAQUA research projects has been very broad, and one area that The Freshwater Institute has been involved with is investigating disinfection practices in RAS; collaborating Nofima scientists have included Drs. Carlo Lazado, Lill-Heidi Johansen, and Åsa Maria Espmark. In particular, one CtrlAQUA study led by The Freshwater Institute’s Research Support Specialist Christine Lepine has focused on the impacts of PAA application on biofilter performance, specifically fluidized sand biofilters, which are more common in U.S. RAS facilities than in other countries.
In support of this research avenue, Lepine led the on-site construction of twelve replicated experimental-scale fluidized sand biofilters (MAHI International, Indianapolis, IN; Figures 1 & 2) which received water from a nearby semi-commercial scale RAS as a source of ammonia-nitrogen. Once nitrification in each biofilter was established, PAA was first assessed by quantifying its decay kinetics following a single pulse application, with target doses ranging from 1.0 mg/L to 2.5 mg/L; the commercial PAA product used consisted of 15% PAA and 10% hydrogen peroxide.
It was observed that, under the study conditions, approximately 50% decay occurred by 10 minutes post-application, and that most (if not all) PAA had decayed by 30 minutes. Biofilter performance post-treatment was assessed via total ammonia nitrogen (TAN) removal efficiency, i.e., the percentage of TAN in the biofilter effluent water relative to the influent water TAN concentration. No significant differences in TAN removal efficiency were observed at 1, 3, or 5 days following the initial pulse dose of PAA, regardless of initial PAA dose concentration.
A second study was performed to mimic more closely a PAA application in a commercial RAS setting, specifically by providing a semi-continuous dose over a 4-hour period with the objective of maintaining recirculating PAA concentrations at target levels for an extended period of time. Target concentrations were low (1.0 mg/L), medium (2.0 mg/L), and high (2.5 mg/L). It was noted during the 4-hour application period, a stable concentration of PAA at the low dose was achieved at around the 25-minute mark, whereas both medium and high PAA concentrations did not plateau until close to the end of the 4-hour period. At 1- and 3-days post-PAA application, no significant differences in TAN removal efficiency were noted. Biofilter effluent nitrite-nitrogen (NO2-N) concentrations, however, were significantly higher in the high PAA dose treatment group at 3- and 5-days post-treatment, although concentration observed were still within a safe range for fish health.
The experiments performed by Lepine et al. demonstrated that, under study conditions, low-dose PAA applications (both pulse and 4-hour semi-continuous) at concentrations that might normally be used to treat waterborne opportunistic pathogens (i.e., 1.0 -2.5 mg/L) did not result in significant disruption of fluidized sand biofilter nitrification performance. This finding is similar to observations in European RAS facilities that typically use different biofilter types. Further research in this area should confirm efficacy against target pathogens at these PAA concentrations, as well as assess longer-term impacts on biofiltration following repeated PAA applications under a range of RAS water quality profiles.
Print this page
Advertisement
- Benchmark Genetics Chile names new general manager
- EAS honours pond aquaculture veteran with life membership award





