
Features
Recirc
Research
Ozone for freshwater RAS
A research summary from the Freshwater Institute
January 16, 2020 By John Davidson
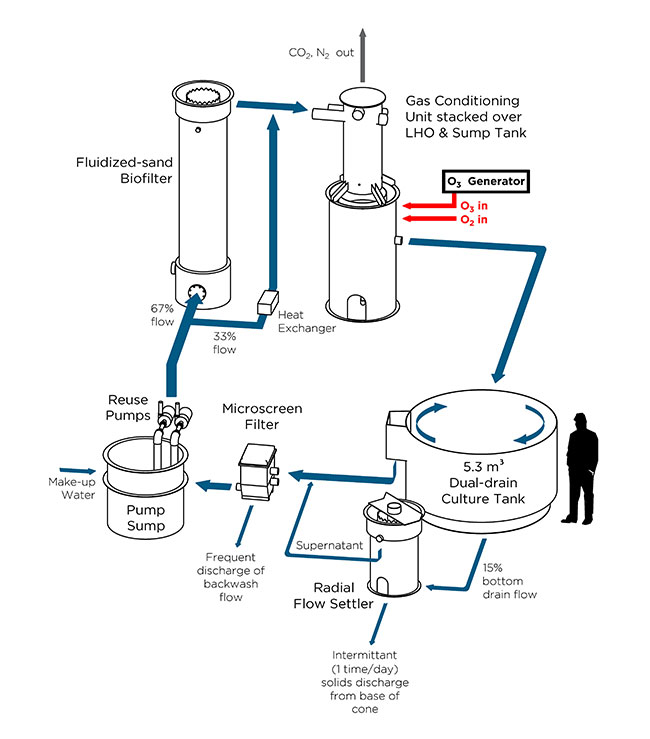 Figure 1. Process flow diagram of a replicate experimental scale RAS showing the point of ozone addition at the low head oxygenator. (All images courtesy of TCF Freshwater Institute)
Figure 1. Process flow diagram of a replicate experimental scale RAS showing the point of ozone addition at the low head oxygenator. (All images courtesy of TCF Freshwater Institute)
Ozone (O3) is a powerful oxidant that provides considerable water quality and fish performance benefits when applied within recirculating aquaculture systems (RAS). Ozone can be used in seawater and brackish water systems. However, its application is safer in freshwater where the primary reaction by-product is oxygen.
Various methods are used to transfer ozone to RAS water, including sidestream contact chambers, fine bubble diffusers, and combined use with foam fractionation. The Conservation Fund Freshwater Institute (FI) has developed a different approach, where ozone is added alongside the primary oxygen gas within a low head oxygenator (Fig. 1). With this method, the entire RAS flow is ozonated just before water returns to the fish tank.
Several precautions are taken to prevent toxic ozone residuals from reaching the fish when using this strategy. First, ozone is applied at a conservative, non-disinfecting dose that is primarily intended for water quality enhancement. As an additional failsafe, oxidation reduction potential (ORP) – an indirect measure of ozone residual – is monitored via a differential sensor positioned at the tank inlet. Millivolt readings from the ORP probe are received by a digital control system that is integrated within an on/off feedback loop. When an upper ORP setpoint is reached (typically 300-320 mV), one of two strategies are employed to maintain a safe ORP level in the fish culture environment: 1) a solenoid valve closes to temporarily suspend ozone addition, or 2) O3 output is automatically discontinued by the ozone generator until a lower ORP setpoint is reached. These processes have been developed and refined over nearly three decades of research and are used in FI’s semi-commercial scale RAS for production of market-size salmonids.
Ozone research
The Freshwater Institute was a pioneer in the development of ozone use in freshwater RAS, with initial research beginning in the 1990s (Bullock et al., 1997; Summerfelt et al., 1997). These early studies found that application of low-dose ozone (̴ 25 g O3/kg feed) reduced the incidence of bacterial gill disease in rainbow trout and improved the fish culture environment by reducing total suspended solids (TSS), total organic carbon, true color, and nitrite-nitrogen levels. Improvements to solids filtration removal efficiencies were also realized.
Subsequent research focused on advanced oxidation of RAS water using high-dose ozone followed by ultraviolet (UV) irradiation (Summerfelt et al., 2004; Sharrer and Summerfelt, 2007; Summerfelt et al., 2009). This research indicated that a relatively strong ozone dose to achieve > 375 mV ORP could be applied to RAS water when followed by UV irradiation levels sufficient to destroy residual ozone before water returned to the fish tank. This approach resulted in several LOG reductions of heterotrophic and coliform bacteria counts.
Although effective disinfection and water quality enhancement were achieved with the ozone/UV method, this design required significant energy to treat the full RAS flow with a UV dose > ~50 mJ/cm2. Over the last decade, FI has continued to expand the knowledge base regarding ozone use in RAS through applied research. Recent trials have increased our understanding of water quality enhancements provided by ozone, and have revealed some interesting trends that point to a possible growth advantage for rainbow trout and Atlantic salmon.
- Experimental RAS operated with ozone controlled at 310-320 mV ORP (left)
- versus RAS operated without ozone (right) while culturing post-smolt Atlantic salmon.
Production in ozonated RAS
In 2011, FI published an article in Aquacultural Engineering overviewing the effects of ozone on water quality and rainbow trout performance while operating RAS at various flushing and feed loading rates (Davidson et al., 2011). Low-dose ozonation resulted in significant reductions in TSS, biochemical oxygen demand, and true color of the culture water. The most profound effect was diminished color, which was otherwise observed as tea-colored turbidity resulting from dissolved organic matter (Fig. 2). Ozone also reduced dissolved metals, including copper, iron, and zinc concentrations. Under certain conditions, total ammonia nitrogen and nitrite-nitrogen were oxidized, and in all cases, ozone increased ultraviolet transmittance of the tank water. Most importantly, this research found that rainbow trout grew significantly faster in low exchange RAS (~7-day HRT) operated with ozone compared to trout raised in similarly operated RAS without ozone (Fig. 3), and without negative effects to key fish health and welfare indicators (Good et al., 2011). A second study indicated that rainbow trout grew similarly in ozonated RAS operated with 1/10th the dilution rate of reference systems without ozone.
Interestingly, a recent unpublished study evaluating the effects of ozone on post-smolt Atlantic salmon performance reflected many of the same production system and fish performance advantages previously observed with rainbow trout. For instance, Atlantic salmon growth curves began to separate immediately after the onset of ozonation and gradually diverged over the next eight months (Fig. 4). It’s likely that cumulative water quality changes brought about by ozone led to enhanced growth during each of these trials, but the exact mechanisms for improved fish performance are unknown.
Ozone limitations
Although ozone provides a wide range of water quality and fish performance benefits, FI’s research has also demonstrated that ozone is not the solution for all RAS challenges. For example, low-dose ozone did not significantly reduce common off-flavor (geosmin and MIB) levels in RAS water or rainbow trout flesh (Schrader et al., 2010). Additionally, while a recent study indicated that ozone could reduce waterborne sex steroids (estradiol, 11-ketotestosterone, testosterone – Good et al., 2017), follow-up research (unpublished) found that ozone did not decrease hormone concentrations to an extent that inhibited early Atlantic salmon maturation. Furthermore, ozone is toxic to both fish and humans at relatively low concentrations. The 8-h human exposure limit for airborne ozone gas established by OSHA is just 0.1 ppm, and the 15-minute exposure limit is only 0.3 ppm. Therefore, it’s highly recommended that RAS facilities utilizing ozone install and maintain ambient ozone sensors, alarms, and remote generator shut-off systems to ensure worker safety.
Finally, ozone generation technology is relatively complex and requires trained fish culture personnel and maintenance staff that are capable of effective operation, maintenance, and equipment repair. Ultimately, RAS facilities must consider the water quality and fish performance benefits that ozone provides along with capital (ozone generators, monitoring systems) and operating (energy, oxygen) costs, and the associated risks to employee and fish health.
John Davidson is a research scientist at The Conservation Fund Freshwater Institute in Shepherdstown, West Virginia, USA.
Print this page
Advertisement
- Hatchery upgrades on the agenda in New York’s $3B environment-focused funding plan
- Philippines authorities explore options to increase supply of milkfish fry







